Medical device prototype development presents critical challenges for MedTech companies. Common issues include inadequate material biocompatibility, structural designs that fail sterilization requirements, and traditional manufacturing limitations for intricate minimally invasive devices. Moreover, collaborating with firms less experienced with medical quality systems such as ISO 13485 can become a serious risk factor with respect to fast approval and time to market. For this project, it shall be assumed that prototypes have standard components with no familiarity with medical standards.
However, a solution for these challenges under regulation, sterilization, and biological safety in design will be explained in a completely different way. Frankly speaking, a solution for these challenges in incorporating regulation, sterilization, and biological safety in design will be uncovered in this stage. The next tutorial will be dealing with a complete solution right from inception to verification. Let’s have a complete view of this tutorial so that we will be able to see how companies like partners LS Manufacturing can assist in working out a prototype based on complete requirements with regards to quality, regulation, and rapid prototype development with ISO 13485 certifications, a clean room, and experience in this area.
| Section | Key Content |
| Introduction | ATT snel: Biocompatibility, Sterilization, Micro-features, & Regulatory Delays Symptoms; Core Problem: Treating the prototypes as standard parts solution, A framework for complaint development |
| Regulatory Foundations | ISO 13485, Quality Management System; ISO 10993, biological compatibility; ISO 11135/11137, sterilization. Demand: Designing for regulatory requirements right from inception. |
| Material Selection | ISO 10993, Sterilizability, Mechanical/Chemical Properties Materials Used Medical Grade Silicones, PEEK, Titanium, Stainless Steel |
| Process & Manufacturing | Micromachining, micro-molding, laser ablation, 3D printing biocompatible. Emphasis: Precision in micro-features, clean |
| Design for Sterilization | Methods: Ethylene Oxide (EtO), Gamma, E-beam, Steam. Design rules: Avoid entrapment zones, material compatibility, consider residual management. |
| Partner Selection | ISO 13485 certification, clean room facilities, experience in medical projects, DFM support, regulation awareness. |
| Path to Success | Steps to Success Requirements need to be imbedded early; then a qualified partnering manufacturer needs to be selected, followed by design for manufacture and sterilization iteration and documenting. |
Successful medical device prototyping requires integrating regulatory and manufacturing requirements from the start. This guide outlines a framework focusing on material biocompatibility, design for sterilization, and precision manufacturing for micro-features. The critical differentiator is partnering with a supplier like LS Manufacturing, which possesses ISO 13485 certification, cleanroom production, and deep medical industry expertise. This ensures prototypes are not just functional but are developed with the compliance, quality, and documentation necessary to accelerate verification, regulatory submission, and ultimately, a faster path to market.
Why trust this guide? Practical experience from LS Manufacturing experts.
In consideration of this information being given in a manner where medical devices are being produced in reality rather than a theoretical setup, our team of prototype designers in medical devices learn something each and every day in our never-ending struggle in achieving a level of accuracy in our implants measured in microns, a level of biocompatibility with each cut given to our devices. The guidelines of testing requirements are being put into heavy-duty implementations, right from design in PTC (Creo) to a high level of prototyping in 3D Systems.
Our experience manifests in over 50,000 PMP machined parts being delivered. Micro surgical instrumentation prototypes for load-bearing devices have given a way to prove our education in achieving a balance in this sense. Every project brings a level of increased experience in our segment with regards to performance, processing, and assemblies in connection with different materials and sterilization.
You can rely on this guidance as it reflects the exacting standards we uphold for every client. By strictly adhering to the engineering principles embedded in PTC (Creo) and leveraging the advanced manufacturing capabilities of partners like 3D Systems, we build prototypes that are not just models, but functional, high-fidelity solutions. Our prototypes are prototypes towards an objective and not scale models. We would like to share our knowledge in this project and definitely bring a change in your project.

What are the key regulations and standards that medical device prototype development must follow by 2025?
one of the most critical standards will be obtained by working on medical device prototype technology. However, the year 2024 will be a very critical year with a simple standard being the last step but not an elementary premise fundamentally incorporated since inception. The 2025 medical device prototype guide will show them the way towards the basic standards, which shall see this prototype move from an elementary premise to a prototype.
QMS/ ISO 13485
ISO 13485, therefore, from prototype production standards, will focus on outstanding design control, risk, and record management. ISO 13485 basically offers a certification standard with regards to all sides of a prototype device model, from an idea to prototype device production, being considered in a controlled manner in a bid to attain traceability uniformity in inspection readiness. ISO 13485 in prototype device technology practices will definitely be among winners in a competition among medical device inventions if put into practice early.
ISO 10993 : Biological Safety Evaluation
The non-compromising part in all cases will never be compromised in a series because medical devices which come into direct and/or indirect bodily contact with devices in a series will include a series such as tests for biocompatibility in a manner which defines a risk assessment of all parts in a prototype device model. ISO 10993 in prototype device model design planning takes into consideration expensive rework in devices, a requirement with government bodies such as FDA.
FDA 21 CFR Part 820/ QSR
The standards do not have a reduced standard for design control. The Complete Design History File is mandatory from the very start in prototype designs. The Design History File shall contain all design inputs and outputs thus providing an audit trail available to support a systematic approach in developing a device design before an actual device will be available in an environment. Equally important, in essence, and to a start-up organization too, is this organization.
Traceability & Process Validation
The prototype will focus on traceability with regards to devices and materials. Here, traceability with regards to an overall prototype with device elements and another one concerning processing devices and tests, which will be traced. Furthermore, in a philosophy with aims on validation targets, in particular with regards to critical process steps concerning handling a device for making devices, you will conclude in ensuring a prototype manufactured in a strategic manner with regards to future massive production.
Furthermore, your medical device prototype development will be among the other medical devices used in productions; hence, your medical device will be developed in this generation. ISO 13485, ISO 10993, and Design Controls are strategic bases among others since, in this case, they are not an organizational challenge for implementation in your medical device prototype based on your 2025 medical device prototype guide.

What are the main processes involved in prototyping medical devices? How to choose the appropriate process based on the stage?
Based on this background, a relevant selection in prototyping will therefore be observed to be an efficient means in prototype manufacturing for medical devices. Every prototyping technique will have strengths and weaknesses up to a level where they will be greatly affected in view of the level of project development in terms of conception level and functionality level of developing medical device prototypes. A guideline will therefore follow in a comprehensively detailed manner where the technique will be linked to rapid prototyping medical devices in a 2025 medical device prototype guide.
| Process | Key Advantages | Limitations/Considerations | Best Application Stage |
| 3D Printing (SLA, SLS, MJF) | Rapid with complicated geometries–even roughing out; visualization models, guiding models. A variety of materials; biocompatible materials available. | Poor mechanical strength; anisotropic properties; may need surface treatment. | Accept/ Prototype Printing, Form & Fit Validation, Anatomicall |
| CNC Machining | Highly accurate & rugged, based on production materials such as commodity metals & PEEK. Good surface finish & tight tolerances. | Exorbitant cost in case of complex shapes. Tooling is inaccessible in design & development stage. Lengthy setup time. | Functional Prototyping & Performance/ Fatigue Testing Pilot production |
| Silicone Molding (Urethane Casting) Production | Unit quantities: 10 to 50 units; detail work can be reproduced. | Small Batch Clinical Trials; Units User Feedback Study; Units Pre-Pilot Production | Small Batch Clinical Trials; Units User Feedback Study; Units Pre-Pilot Production |
Medical device prototyping can be described in terms of saying, which starts with, “it is a matter of coordinating designs with production technology.” In rapid prototyping, if one were to talk in terms of rapid prototyping, 3D printing eclipses all other aspects in fast prototyping. Next, in a prototype with a better degree of durability in a practical prototype testing, all other matters will have to do with CNC machining. Finally, in medical simulation where a final product in terms of medical simulation using a near-final product prototype is employed, a better in-between in prototyping medical devices would be silicone molding.
How to select prototype materials for medical devices that meet biocompatibility requirements?
Materials selection is a factor in medical device prototype. The materials used in medical prototypes will not only have specifications in relation to mechanics, but they will also have to be biologically safe since medical prototypes will have established biocompatibility. Making the correct choice early in the prototype manufacturing for medical devices process is critical to avoiding costly redesigns and ensuring patient safety.
| Material Type | Raw Materials | Primary Advantages & Typical Applications |
| Polymers | Med-2 medical-grade PEEK, PC-ISO, UHMWPE, USP VI Silicones | Very suitable when used in medical disposable products, containers, wearables. Characteristics include a good level of biocompatibility, resistance to sterilization processing, other special properties such as radio-opacity, or flexibility. |
| Metals | Titanium (Ti-6Al-4V ELI), Stainless Steel (316L), Cobalt-Chromium Alloys | The LS Manufacturing prototype should be produced in a way that it is strong with abilities to withstand fatigue and have capabilities of osseointegration. |
| Other | Bio-compatible 3D Printing Resins (e.g., Dental SG, MED610) | These resins allow rapid prototyping of complicated anatomic parts, surgical guides, and fit devices. |
Ultimately, material selection is an absolutely strategic step in prototype manufacturing and a well-rounded action with and in regulatory science. Having companies such as LS Manufacturing on your side can become a very important element in your project because not only will they give you access to approved amounts of materials with traceability, but they will also provide a skill set which will allow you to direct your selection into your prototype device in terms of class, time, and sterility.

How does design for manufacturing affect the success rate and cost of medical device prototypes?
While principles of design for manufacturability can be embedded in a step of a manufacturing process, this guiding principle is very important when one finds oneself medical device prototyping. A proactive stance with respect to such a guiding principle of a step in DFM/DFA in a prototype in medical devices basically constitutes a guiding principle in the 2025 medical device prototype guide in order to suggest cost in terms of both manufacturability and success.
Optimize Production Process
Prototype design optimized for production shall address manufacturability from inception. The considerations include a prototype design where all wall thicknesses are equal in an injection molded prototype design, or paths in a CNC prototype design. Prototype design optimized for production will our production prototype end up being a scalable design.
Sterilization & Compliance Design
Sterilize a prototype. Some of the considerations with respect to DFM will remove all crevices, corners, and blind holes of a device which have the potential of damaging a sterilizer in a device or creating a bacteria culture site. Evidently, in the foreseeable future, prospects in medical device prototype design with respect to an assessment relating to the design stage of a device, a problem in design in a sterilization validation stage will have a potential to cause serious delays in a project or tooling modifications.
Simplified Assembly and Tolerancing
DFA will make part complexity and intricacies in an assembly simplified. Very minimal involvement, and more importantly, it will do this in a wise definition of tolerance. A wise definition of tolerance will not allow a very tight definition of a product since it will make the cost of prototype production very expensive to the level where a loose definition will impact the functionality of the product.
In other words, incorporating a DFM/DFA mindset is narrowly put as that which differentiates a prototype from being functional to one that lays a solid foundation for market launch. Being at the leading edge of 2025 medical device prototype guide, this mindset will be important in ensuring that medical device prototype design are not just based on investment but rather manufacturability, regulatory, and cost-of-manufacturing. Through this, they can benefit from incorporating DFA expertise early to mitigate risks and thus quickly deliver a prototype rather than a model.
Case Study of Titanium Alloy Structural Components for Minimally Invasive Surgical Instruments: How LS Manufacturing Achieves Precision and Biocompatibility
Success with the more complicated device relies upon developing a good working relationship with a suitable company, which not only possesses capability but can address such harsh requirements. The above case study also illustrates how a specialized approach in LS Manufacturing relieved an important bottleneck in medical device prototype development in order to obtain a part with accuracy in micron-level accuracy, yet with complacency in biocompatibility.
Client Challenge
The problem that therefore needed a solution in this case for our company was how to treat a minimally invasive surgical device developed by one of our clients. Their project had a very critical part with a very small tolerance requirement of a titanium alloy of just ±0.025mm, and another critical part for which biocompatibility requirements existed. Suppliers were not very easy to come by, since a demand existed for this very same expertise in medical-grade titanium machining, with controlled clean room environments conducive to competitive part production on which prototype manufacturing for medical devices could be attained.
LS Manufacturing Solution
Our team developed a protocol with a medical-grade project focus. As a team, therefore, our methodology did prove to have established an implant-grade Ti-6AL-4V ELI alloy with traceability; machining in controlled environments did prove to have occurred. With this machining achieved in a 5-axis CNC machine, our problem solution requirement did prove to fulfill requirements because of machining accuracy. Every step in being accomplished in machining and post-processing in machining did prove to have all steps accomplished in regards to cleaning under our ISO 13485 Quality System requirement in order to have our LS Manufacturing prototype unstained but ready for initiation of biologic tests.
Results and Value
Our prototype parts met all the requirements for specifications concerning dimensions, specifications for surface finish, and tests for biocompatibility concerning requirements under ISO 10993. They were capable of immediate use directly in critical animal studies, which essentially helped our clients fulfill their FDA approval requirements. Our approach to meeting this basic requirement among medical device prototype firms immediately accelerated the overall medical device prototype development time schedule by a total of about 30% in essence, proving the effectiveness of ours right at this early stage of prototypes.
Other principal aims in this case include our role in substantially testing our premise with respect to medical device prototypes in our role in ‘stands’ or ‘standpoints’ with this: “Attention to medical detail in medical prototype machining will have medical-grade quality requirements in the development of medical device prototypes. Our projects for LS Manufacturing prototype can influence your medical device prototype’s feasibility in medical markets.”
Do you have your medical prototype in a rut due to your medical device prototype complying requirements? Then contact our Medical Project Professionals at LS Manufacturing today!

What is the typical timeframe for a medical device prototype from design freeze to delivery?
Predictability of time in medical innovation is a whole lot of significance. A scale of time, which begins right from when a prototype design gets ‘frozen’ to when it gets delivered with a medical device prototype, can have an entire gamut of variables. Moreover, this whole lot of variables can be determined by a whole load of important factors rather than getting stuck into a fixed figure. With this 2025 medical device prototype guide, this article will attempt to focus on these variables and will attempt to present a reality check with regards to medical device prototype project planning and rapid prototyping medical devices.
Design Complexity & Process Selection
Process selection in product production can be stated to be most critical & time-consuming in this case. Therefore, a prototype with rapid prototyping & 3D printing can be accomplished in no less than 1-3 working days with idealism, but a functional prototype with multi-axis CNC machining of biocompatible metals will take up to the longest time span of 2-4 weeks since a whole lot of jobs need to be accomplished to reach a level of acceptability fit for a medical prototype.
Logistic Material and Compliances
Sourcing certified, traceable medical-grade materials (e.g., Ti-6Al-4V ELI, PEEK) adds procurement time compared to standard industrial grades. Any downstream processing, other than a function of passivation, specialized cleaning, and/or pre-processing, will bring in a time factor. The same downstream processing will not be negotiated during the course of developing a medical device prototype.
Highly efficient inspection
It includes complete FAI with dimensional checks, which is a very time-consuming but important function of design verification. For this step, a variety of partners work in parallel for operations of workflow because this will allow them to quickly document quality in sync with production, with quick prototypes and with an optimized medical-grade material supply chain.
To conclude, based on this article, if you were working on a medical device prototype, you could look forward to, hopefully, completing a medical device prototype in 1-4 weeks based on complexities. Based on quotes presented above in this tutorial on 2025 medical device prototype guide, projects, processing, medical requirements, and medical complexities appear to have a higher level of importance in rapid prototyping medical devices, which in essence is a main factor in completing your medical device prototype.
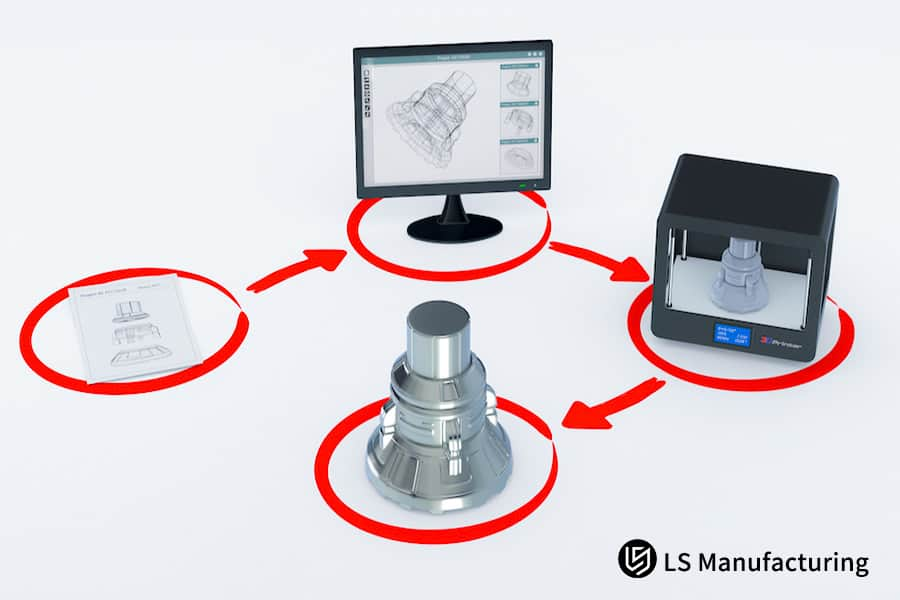
What core qualifications and capabilities should be examined when evaluating a medical device prototype manufacturer?
Your choice of prototype manufacturer is very strategic. They do, in fact, impact your entire developmental stage based on the level of compliancy and quality level, in addition to time frame. Your essential requirements are very unique in this given case, and you have a set of requirements which have to be fulfilled when selecting a suitable provider in this case, since you are able to think of them as an extension of your team in searching for a suitable medical device prototype manufacturer.
- Formal Quality System Certification: In this case, ISO 13485 certification will be mandatory. As stated in this case, therefore, an equal standards prototype in regards to a commercial device prototype will therefore have to be developed right from the onset of this idea, in this, the first LS Manufacturing prototype.
- Sparrow Production Environment Assessment: A medical device prototype manufacturer will thus have to have a clean room with a good working environment if they were to avoid any form of contamination. They will thus become important in regards to processing medical materials which they will be able to trace. They shall not compromise in any way with biocompatibility, right from their inception.
- Full Inspection and Documentation Capability: Precision fabrication has to be at par with precision verification. They will have to be asked for their capabilities in house in metrology, quality process with capability to provide complete inspection report, certification, and traceability documents for each prototype manufacturing for medical devices, which will have to be in order to support your design verification and your due diligence research.
- Relevant and Proven Project Experience: Direct experience with devices in a similar class and application is invaluable. A manufacturer with a portfolio of successful LS Manufacturing prototype projects for Class II or III devices brings practical knowledge of regulatory pathways, testing requirements, and design for manufacturability, offering strategic guidance beyond simple part fabrication.
In case of a medical device prototype manufacturer, a broad perspective with respect to certifications, controlled production environment, medical-grade material mastering, and project experience in medical devices which have already been established in medical device projects in the past is a critical point to be considered. A prototype manufacturer such as LS Manufacturing, which scores better in all these aspects, will not be part-producing but will deliver core with quality, which will fast-track your entire product developmental life cycles.

What is the process for collaborating with LS Manufacturing on a medical device prototype project?
The engine of prototype development is an entirely compliant processing system in a transparent and clear manner. Our systematic medical device prototype collaboration process is designed to be predictable with improved quality assurance and risk management in partners, which will fast-track your product development life cycles. Medical device prototype development with complicated designs can be split into a series of manageable projects.
- Project Initiation & NDA: Every project starts with a talk with a common Non-Disclosure Agreement. An early step towards a profound understanding of your design application, approach, timeline, and following profound understanding for developing your medical device prototype, but in order to clarify, a common vision and a trust-building platform is established for this full medical device prototype development project.
- Technical & Regulatory Design Review: A thorough step performed by our engineers & quality department in accordance with manufacturability, biocompatibility, and sterilization compatibility of your design. This proactive review integrates compliance from the outset, identifying potential issues early to prevent costly revisions later in the medical device prototype collaboration process.
- Formal Proposal & Project Plan: Furthermore, you will be provided a fixed scope proposal with a project schedule where all our pricing is presented neatly in a project schedule, with a discussion on project risk management in place. Acceptance of this project unleashes our project work and brings all project participants into project expectations among them based on your LS Manufacturing prototype project requirement delivery.
- Control Manufacturing Execution: Next, production-ready designs will be manufactured in our controlled ISO 13485 environment. With our latest technology such as our machine shop with advanced CNC machining in our ISO 13485 controlled production environment, our production of your medical device prototype will construct your prototype with very controlled specifications, traceable with our part traceability in medical-grade materials.
- Extensive Inspection & Medical-Grade Cleaning: Our dimensioning and inspection process is a very thorough check in our projects for dimensional and visual inspection complete in our projects, with jobs involving advanced coordinate measuring machine technology in our advanced analytical lab. Having performed this inspection, our prototypes will undergo a validated clean where all remnants of our manufactured product are eliminated from our product-manufacturing process; prototypes will be ready for your function tests or biocompatibility tests.
- Delivery & Complete Documentation: With this, you will be given not only your prototype delivery but will have all your prototype documents delivered too. Inspection report will include an inspection report with additional specifications, materials certification, and conformity to certificate. Moreover, this inspection report is a vital delivery requirement and used in order to facilitate your vital medical device prototype service in your entire medical device prototype development cycle.
A six-step medical device prototype collaboration process will be described below with a definite gating protocol in each step with our medical devices. “The model is one where detail matters eliminate uncertainty in medical device prototype development, deliver faster results, and provide you with an LS Manufacturing prototype which will definitely not only work but is a critical step in your journey towards approval.”

FAQs
1. Do you possess an ISO 13485 accredited quality system?
Because LS Manufacturing has been ISO 13485: 2016 certified for quality management systems. Based on your requirement about the medical device in regard to quality control standards, right from the design and development stage up to the prototype stage, we work in line with your requirements to cover your whole need for the medical device.
2. Biocompatibility tests, if prototype materials are available.
USP Class VI or ISO 10993 series specifications requirements for all types of raw medical grade materials include detailed reports of biocompatibility tests. Alternatively, support documents shall establish proof of fulfilling specifications in case the materials used will meet them after a biologic evaluation.
3. What’s MOQ of the medical device prototype?
This is about on-demand research and innovation development, flexible design prototyping without considering the least quantity of the order, a single prototype, and small batch verification. These are going to be some of the ways we will be able to help in your product design developments with an added degree of efficiency.
4. How long does it take to get a full quotation?
Based on prototype projects, as for the examples of medical device projects, if you are dealing with a prototype project, it is possible to provide a complete solution-analyze our process within 1-2 business days with your quotation. The end product of everything will be an ability to give you a fast solution so your project can be launched immediately.
5. How do you go about protecting your technical writing and IP with respect to your prototype designs?
Of course, all our work is carried out in complete accordance with our NDAs. Our technical documents are transferred and stored in an encrypted system with the access control system. Much attention is given to safety and with concern for our client’s IP rights, taking into consideration the requirements of information safety within the medical community.
6. What class of room w.r.t. production is any clean room available?
At the moment, our company currently makes use of just one ISO class 8 clean room, Class 10 000 rated. The clean room is, in general, a space with controlled particulates and microorganisms with an objective of providing a clean environment for a medical prototype.
7. How to start with a very first prototype project in medical devices for new customers?
Every new customer can freely contact our website regarding our medical project, or come up with another form of direct contact. Further, if they have an order and this order is in a developmental stage, they will have to have an assigned engineer for an individual service in order to help them when developing their very first prototype.
8. Can you undertake small batch production for pilot tests, based on prototype development?
Prototyping and small batch production are available, which may include prototyping validation and prototyping optimization in some instances within your design to ensure time to market.
Conclusion
Medical device prototyping can be that important interface to bridge the gap between an innovative design and a successful medical device. Your quality and standards in the medical device prototype, along with time-bound prototyping performance ability, will definitely impact your level of success in medical device prototyping. You are, therefore, supposed to undertake all the necessary steps so that you will be able to find a collaborator with precision manufacturing experience and knowledge of the rules and regulations.
Let’s begin your compliant and effective medical device prototype project today! Just click “Get Free Medical Device Prototyping Technical Consultation“. We will shield your medical invention at LS Manufacturing to make it professional.

📞Phone:+86 185 6675 9667
📧Email:info@longshengmfg.com
🌐Website:https://www.longshengmfg.com/
Disclaimer
The content appearing on this webpage is for informational purposes only. LS Manufacturing makes no representation or warranty of any kind, be it expressed or implied, as to the accuracy, completeness, or validity of the information. Any performance parameters, geometric tolerances, specific design features, quality and types of materials, or processes should not be inferred to represent what will be delivered by third-party suppliers or manufacturers through LS Manufacturing’s network. Buyers seeking quotes for parts are responsible for defining the specific requirements for those parts. Please contact to our for more information.
LS Manufacturing Team
This article was written by various LS Manufacturing contributors. LS Manufacturing is a leading resource on manufacturing with CNC machining, sheet metal fabrication, 3D printing, injection molding,metal stamping and more.






