The medical market places extremely strict demands on plastic components, not just for biocompatibility, dimensional stability, and cleanliness, but also directly impacting patient safety and treatment performance. Medical injection molding is a core manufacturing technology that responds to such strict demands. Due to its high accuracy, high reliability, and good sterilization capability, it has become a critical process in the production of medical devices.
In the following article, this process will be systematically discussed in terms of its special benefits and extensive use and will show how, as a professional custom medical device manufacturer, LS Precision Manufacturing offers customers a one-stop solution from conceptual design to mass production by means of complete medical injection molding services.
At LS Precision, medical injection molding is more than manufacturing; it’s a commitment to life. We adhere to the highest possible standards to ensure that each part is safe and reliable. To save your time, take a quick glance below at the key takeaways.
Medical Injection Molding Core Quick Reference
| Core Aspects | Key Summary |
| Core Values | Medical injection molding stands out as the top preference for medical device production due to its high precision, efficiency, good biocompatibility, and mass production consistency. |
| Production Environment | Production takes place within high-grade cleanroom (e.g., ISO Class 7 and above), with strict control of microorganisms and particulates, ensuring product sterility and safety. |
| Material Selection | Biocompatible USP Class VI and ISO 10993-approved materials are used to ensure safety and reliability, meeting regulatory and performance criteria for use in the medical environment. |
| Process Monitoring | Closed-loop process monitoring is implemented throughout the process from mold design through precision injection molding, automatic assembly, sterilization, and packaging. Traceability is ensured by a zero-defect traceability system. |
| Core Competencies of an Excellent Service Provider | LS Precision provides one-stop services from prototype to mass production, from mold to certification, saving customers risk and reducing product time to market. |
| Transition from Prototype to Mass Production | With prototype testing, process qualification, and limited-batch pilot manufacturing, meticulous checking ensures design stability and process maturity, enabling convenient and seamless transition from prototype to big-batch mass production. |
Medical injection molding is a sensible project composed of precision craftsmanship, severe environment control, advanced material science, and strict quality control. Its vital role is to produce medical devices safely, steadily, and economically according to strict medical standards.
Choosing a partner with full capacities and profound experience like LS Precision is the first step to driving projects from design to market successfully and achieving zero-defect mass production.
Why Trust This Guide? Practical Experience From LS Experts
This guide is the result of 10 years of technical expertise and medical injection molding experience of LS Precision Manufacturing. We have over 200 precision machining and testing machines, as well as a Class 100,000 cleanroom and Class 10,000 laboratory, which gives us the manufacturing facility for strict medical specifications. In quality management, the company is both ISO9001 and ISO13485 certified and has made investments in ERP and MES systems with a vision of achieving digital and intelligent process control of manufacturing.
LS Precision is an expert company in liquid silicone rubber (LSR) overmolding technology with micron-level tolerance control capability and has been successfully utilized in high-precision medical components such as high-end surgical instruments. Following the philosophy of “Precision, Intelligence, and the Future” of development, we continuously update new material R&D, process optimization, and expanded application, providing higher-quality more accurate and efficient manufacturing solutions to customers worldwide.
Why Is Medical Injection Molding The Preferred Method For Medical Device Manufacturing?
Product quality, as it is quantified in terms of product safety, reliability, and consistency, is most important for the manufacture of medical devices. Medical injection molding, as it possesses strongly technical features, is increasingly becoming an inevitable key process for the manufacture of medical devices, making the high-standard manufacture of all types of medical devices possible through a strong foundation.
1. Extremely High Repeatability and Consistency:
High-precision injection molding is possible at the micron level, or extremely consistent part quality in high-volume manufacturing. Such consistency is of paramount concern in surgical instruments, implant components, and highly precise diagnostic consumables and has direct bearing on medical device performance and therapeutic safety and therefore is of literally enormous importance in medical injection molding.
2. Significant High-Volume Cost-Effectiveness:
Compared to traditional manufacturing methods such as machining and 3D printing, injection molding is largely cost-effective for mass production. Once the accuracy molds are developed, the unit costs are significantly reduced, and production efficiency is notably increased. This is particularly suitable for molding medical products such as such as catheter connectors and syringes, which are produced in millions of units.
3. Molding Capabilities for Complex Microstructures:
Most of today’s medical devices, such as microfluidic chips and endoscope components, possess extremely complex and miniaturized geometries. Medical injection molding technology is capable of shaping such complex structures in a single stroke, which is difficult or very expensive to achieve using other process technologies, which helps highlight its pivotal role in medical device molding.
4. Wide Selection of Medical-Grade Materials:
It has a wide variety of medical-grade polymer materials, including PP, PC, PEEK, and silicone that meet ISO 10993 standards of biocompatibility, meeting the demanding requirements of chemical stability, radiation, or steam sterilization. This versatility of materials further promotes the advantages of medical injection molding as the best choice for manufacturing different medical devices.
The overall performance capabilities of medical injection molding in precision, efficiency, forming complex structures, and material compatibility make it a top molding technology selection for medical devices.

In What Environment Are Your Parts Made?
Unlike conventional injection molding, medical injection molding needs to be in a precisely controlled environment. This not only ensures precision but also ensures product safety and biocompatibility. Your medical components are manufactured in a higher-grade cleanroom than those in conventional factories.
1. High-Class Cleanroom: Isolating Contamination at the Source
In order to ensure absolute product sterility, true medical injection molding must be performed in at least an ISO Class 7 (Class 10,000) or higher cleanroom. Such an environment uses a HEPA filtering system to constantly control airborne particle and microorganism levels, eliminating the potential for contamination from the very beginning and providing a warranty basis for accurate medical molding.
2. Strict Personnel and Process Control:
At LS Precision, we realize the importance of environmental control. Personnel who enter the cleanroom must be trained professionally and wear cleanroom-specific uniforms, masks, gloves, shoes, and caps to minimize particulate and microbiological contamination from the human body. All materials are cleaned and run through standard air showers or pass-through windows such that all production process steps are in control.
3. The Essential Difference from Ordinary Injection Molding:
This intense emphasis on protecting the environment is the main difference between precision medical molding and standard injection molding. While standard injection molding is prompted primarily by business requirements, precision medical molding responds to the environment as a matter of product quality. The final product often comes into direct contact with the human body or is implanted, and even slight contamination can lead to severe clinical hazards. Therefore, manufacturing in a clean environment with international standards is absolutely necessary to ensure the safety and efficacy of medical devices.
LS precision medical injection molded components are manufactured in ISO Class 7 (Class 10,000) cleanrooms. With rigorous process and environmental control, contamination is effectively eliminated, providing precision and safety. This essentially differs from typical injection molding.
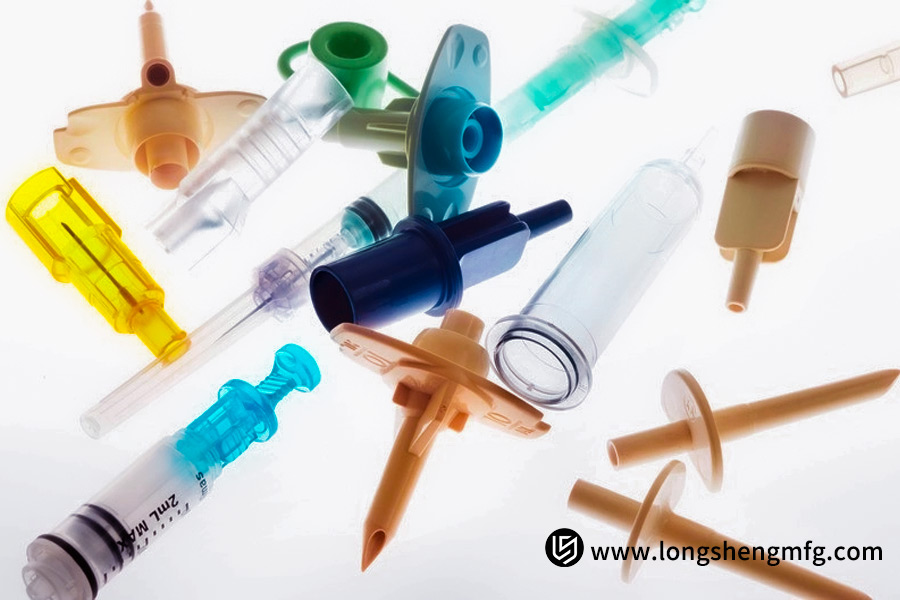
How To Choose “Top-Quality Materials” For Your Medical Products?
Material selection for medical devices is an important decision, with direct consequences on product performance, safety, and regulatory approval. Good-quality materials used for injection-molding medical devices must not only possess improved physical characteristics but also be subjected to extensive biocompatibility testing (e.g., ISO 10993). Some high-quality materials that are widely used in injection-molded surgical instruments and high-end medical devices are as follows:
1. Medical-grade PP (polypropylene) and ABS:
All-purpose engineering plastics are widely used in the casings of in vitro diagnostic (IVD) devices, liquid reservoirs, and other accessories because of their excellent chemical resistance and economic advantages. They generally possess good sterilizability and appropriate compatibility for gamma radiation or ethylene oxide (EO) sterilization.
2. High-performance specialty plastics: PC, PEEK, PEI (ULTEM):
- PC (polycarbonate): With superior clarity and high impact strength, PC is the ideal choice for respiratory masks, clear surgical instrument components, and blood separation plates.
- PEEK and PEI: These two high-performance polymers possess superb strength, heat stability, and resistance to fatigue and can be sterilized repeatedly by autoclaving. They are unsurpassed in applications for long-term implantable devices and high-performance injection-molded surgical instruments, i.e., laparoscopic instrument articulations.
3. Medical Silicone Rubber (LSR):
Liquid silicone rubber offers high biocompatibility, flexibility, and heat resistance, therefore making it the top-of-the-line medical injection-molding material of choice for masks, seals, catheters, and other soft parts in direct contact with human tissue.
The material of choice depends on the final application of the product, sterilization type, and level of patient interface. Based on your specific needs, our LS materials engineers will recommend the optimal solution from our entire catalog of certified materials for guaranteeing the performance and safety of your product.
How Do We Achieve A Closed-Loop Quality System Throughout The Entire Medical Injection Molding Process?
In the manufacturing of medical injection molding, quality is not an at-one-step controlled process, but a closed-loop system from the entire life cycle of the product. The core competitiveness of medical injection molding is such a completely traceable and controllable system of quality management from mold to package.
1. Ultra-high-precision molds:
Molds are the basis of precision medical molding. We use precision machining technologies such as mirror polishing and hard chrome plating to ensure molds not just are dimensionally accurate but also with extremely high surface finish and abrasion resistance. This eliminates defects like burrs and flow marks at the outset, ensuring stable mass production.
2. Rigorous Process Validation (IQ/OQ/PQ) System:
LS Precision rigorously qualify each injection molding process and mold parameter by careful equipment installation qualification (IQ), operation qualification (OQ), and performance qualification (PQ). This means that production officially begins only after the process window has been thoroughly qualified as stable and reliable, ensuring very consistent product performance.
3. Fully Automated Production and 100% Online Inspection:
In the manufacturing process, LS Precision apply robotic automation and clean, sealed lines of manufacture to completely eliminate the chance of human contamination. Moreover, we apply vision inspection tools and laser measuring devices for 100% real-time monitoring of key dimensions, eliminating defective products in real time and ensuring that all products leaving the manufacturing line meet design standards.
4. Clean Packaging and a Complete Traceability System:
Products are packaged under clean conditions, and packaging materials themselves are inspected for the purpose and antibacterial ability. Products of every batch are individually labeled, enabling forward and backward traceability from raw material, production batch, process parameter, through to finished product, creating a complete closed-loop quality management system.
LS Precision established a comprehensive quality management system by integrating profound precision mold technology, standardized process verification, automatic manufacturing, and intelligent inspection.
Not only does this reflect the extremely high demand of medical injection molding technology, but it also ensures precision medical molded products’ safety and reliability, providing customers with perfect quality assurance.

What Other Core Capabilities Should An Excellent Medical Injection Molding Service Provider Possess?
When selecting a credible custom medical device manufacturing business, consider not only its injection machinery but also its ability to provide added value and core competencies from product design through retirement. A true medical injection molding service is a cradle-to-grave solution that integrates technology, regulation, and quality.
1. Independent Mold Design and Manufacturing Capabilities:
Top-tier service providers must possess independent precision mold R&D and manufacturing bases. This means that they can intervene from the initial design-for-manufacturing (DFM) stage in order to guarantee product structure and mold design optimization, manufacturability of parts, high accuracy, and stable production for the long term—the foundation of product quality and cost control.
2. Comprehensive Validation and Testing Service System:
Apart from production, all the validation support must be guaranteed, i.e., verification of dimensional accuracy (through 3D imaging and scanning), verification of mechanical properties, verification of chemical compatibility, and cleanliness control. This system provides scientific evidence to guarantee each batch of products to satisfy strict design specifications and zero-defect shipping.
3. Extensive Regulatory Registration and System Support:
Your firm must have a well-established custom medical device manufacturing firm as its regulatory partner. It should possess an ISO 13485 certified quality management system and be extremely familiar with global market regulatory guidelines like FDA CFR 21. It can provide customers with necessary technical support documents such as DMR and DHF for product registration and prior market entry.
4. Subsequent Sterilization and Packaging Integration Services:
The final critical process involved in medical devices is packaging and sterilization. A supplier capable of offering validated sterilization processes such as ethylene oxide (EO) and irradiation, along with corresponding packaging validation services, will greatly simplify supply chain management for customers and deliver end users safe and sterile product delivery.
Reduced risk, higher efficiency, and less trouble in overall service are the outcomes of an end-to-end solution partner like LS Precision, the secret to project success.

Case Study: How Did We Achieve Zero-Defect Mass Production For A Disposable Endoscope Accessory?
In medical injection molding, it is good proof of a manufacturer’s technology to obtain an ultra-high yield rate for complex structures. Following is our actual case about how we were able to accomplish zero-defect mass production of disposable endoscope accessories for one of our overseas customers.
1. Customer Dilemma:
A global leader among medical device firms needed a new disposable biopsy forceps handle with an ultrahigh-level structure, exacting tolerances, and extremely high surface finish. The then-current supplier had a product yield rate consistently at 92%, which could not match the firm’s zero defect quality goals, low cost, and high volume. The project was highly challenging.
2. LS Precision’s Solution:
As a specialty medical device manufacturer, LS Precision engaged an exclusive technical team to assist in surmounting these hurdles:
- LS Precision began with the middle mold, incorporating specialty steel and symmetrical filling runner design optimization.
- To manufacture the product, we utilized cavity pressure sensors and in-mold hot runner precision control systems, allowing millisecond-level injection molding fine-tuning for absolute mold-to-mold consistency guaranteed.
All procedures are performed on fully automated machinery in a Class 10,000 cleanroom and eliminate contamination by not being in contact with humans.
3. Results:
By leveraging all of the medical injection molding expertise of LS Precision Manufacturing, all-time high levels of performance were achieved on the project: multiple runs of millions of units, yield improvement from 92% to over 99.95% consistently, and an unparalleled record of zero customer complaints. Not only was this a relief for a pain point for the customer, it solidified our status as their strategic, long-term partner of choice.
Do your medical project also demand the best in terms of quality and consistency? Contact LS Precision‘s medical project experts today to begin your journey to zero-defect manufacturing!

How To Seamlessly Transition From Prototype To Mass Production?
Transition from prototyping to volume manufacturing in medical device production is typically challenging. This gap results in consistency problems in the product and late market introduction. LS Precision medical injection molding service has a standard process to ensure smooth transition from prototyping to volume production.
1. Prototype Mold Development Based on Mass Production Standards:
LS Precision uses the same mold steel and process procedures used for mass production to manufacture prototypes. While runners are reduced in prototype manufacturing, the core cavity is shipped with the production mold from the start so that the prototype is identical in size, appearance, and functionality to the end product, paving the way for future mass production of the medical devices.
2. Accurate Verification and Transfer of Process Parameters:
During the prototype stage, LS Precision conducts rigorous process debugging and verification to determine the optimal range of injection molding parameters. The strictly verified parameters are retained in their entirety and directly transferred to the mass production area in order to manufacture extremely consistent products molded by way of mass production by being replicates of the prototype, thus providing smooth medical injection molding services.
3. The Core Value of Seamless Integration: Quality and Efficiency:
Seamless integration method eliminates quality risks and avoids delays caused by re-molding or process changes. The customers not only get samples but also qualified, production-quality solutions, minimizing their time to market. Regarding prototyping as a standard prelude for mass production, LS Precision utilizes scientific process validation and mold technology to achieve a single-step transfer molding from prototyping to mass production of medical devices, offering customers cost-effective, high-quality, and risk-controlled medical injection molding solutions.
FAQs
1. What is the typical tolerance range for LS Precision Medical Injection Molding?
LS Precision’s medical injection molding accuracy can achieve tolerances of ±0.01mm to ±0.05mm consistently. Its high-precision accuracy is customized according to part size, structural complexity, and material properties and is crafted to handle the high-tech demands of high-precision medical devices such as minimally invasive surgical instruments and high-end diagnostic instruments.
2. Do you have relevant medical quality system certifications?
Yes. LS Precision fully implements the ISO 13485 medical device quality management system and strictly conforms to international regulations such as FDA 21 CFR Part 820. We have established a complete product traceability and risk management system to enable the entire process from design to production to meet the access needs of the regulation of the key international markets.
3. Can you accept small-batch pilot production orders for medical devices?
Yes. LS Precision offers full-cycle operations, ranging from prototyping and small-lot trial production to mass production of large volumes. With our experienced process team and multi-functional production lines, we are capable of delivering timely response to each customer order in every step of the product for R&D verification and transitional smoothness to mass production.
4. How do I start my medical injection molding project?
Send us your product specifications or 3D models via email. LS Precision will have an immediate confidential review and analysis, for example, a full project plan with material selection, process design, validation plan development, and a full quotation to help progress your medical injection molding project with simplicity.

Conclusion
Medical injection molding is a highly technical business, requiring precision engineering, material science, and rigorous regulatory standards. It becomes essential to choose an experienced partner with profound know-how, robust technical skills, and rigorous quality control expertise for the successful implementation and effective launch of medical device products.
LS Precision specializes in providing customers with complete medical injection molding solutions that are technologically advanced and trustworthy. Through our precision process expertise, stringently clean compliant production, and familiarity with international quality standards, we engineer each product to the highest degree of quality from design through delivery. If you’re designing a new medical device, your go-to manufacturing firm is LS Precision Manufacturing.
Contact LS Precision today for a complimentary feasibility study for the industrialization of medical devices! Let us offer sound, credible industrialization services for your invention using our expertise.
📞Phone:+86 185 6675 9667
📧Email:info@longshengmfg.com
🌐Website:https://www.longshengmfg.com/
Disclaimer
The content appearing on this webpage is for informational purposes only. LS makes no representation or warranty of any kind, be it expressed or implied, as to the accuracy, completeness, or validity of the information. Any performance parameters, geometric tolerances, specific design features, quality and types of materials, or processes should not be inferred to represent what will be delivered by third-party suppliers or manufacturers through LS’s network. Buyers seeking quotes for parts are responsible for defining the specific requirements for those parts. Please contact to our for more information.
Team LS
This article was written by various LS contributors. LS is a leading resource on manufacturing with CNC machining, sheet metal fabrication, 3D printing, injection molding,metal stamping and more.







