Do you often experience surprise rusting, stubborn weld staining, or contamination on stainless steel processing? These small blemishes actually point to an intrinsic process flaw—poor passivation. Stainless steel corrosion resistance is not inherent; it relies upon a thin but robust film of passivation on the surface.
Misapplied passivation methods, rather than creating this protecting layer, can introduce additional secondary contamination and even make localized corrosion grow, leading to a drastic shortening of product life, failure in function, and finally expensive customer returns and loss of brand name reputation.
It is not hyperbole; one mistake can eliminate the entire value of a workpiece. Consequently, scientific and standard passivation processes cannot be carried out; they are a product quality assurance and financial loss prevention quality process platform of gigantic scale. For the sake of saving your precious time, here below is an instant overview of the major points.
Quick Reference: Choose Your Tap At A Glance
| Key Steps | Key Techniques |
| 1. Thorough Pretreatment | Pre-passivation, gentle cleaning, and degreasing must be performed to thoroughly remove all surface impurities, including oil, coolant, and markings. |
| 2. Cleaning High Carbon/Weld Zones | Heat-affected zones (HAZs), spatter, and heat treatment scale must be ground away mechanically or pickled (nitric acid-hydrofluoric acid). |
| 3. Solution Selection and Concentration | Based on the stainless steel grade (e.g., 304/316 vs. 400 series), employ environmentally friendly solutions such as nitric acid, nitric acid + dichromate, or citric acid, and adhere to recommended concentrations rigidly. |
| 4. Strict Temperature and Time Control | Adhere rigorously to the “gold standard” of solution temperature and immersion time described in the process specification. Use a thermometer and timer. |
| 5. Thorough Post-Cleaning and Neutralization | Rinse after passivation with copious volumes of clean, high-pressure water. Neutralize using an alkaline solution (e.g., sodium carbonate) if required. |
| 6. Complete Drying | Dry the workpiece thoroughly after washing using chloride-free compressed air, a drying oven, or a clean absorbent cloth. |
| 7. Verification | Verify the quality and uniformity of the passivation film through routine tests (e.g., copper sulfate spot test and potassium ferricyanide test), as opposed to visual inspection only. |
Stainless steel passivation is no rapid “acid soak”; it is a specific, step-by-step process. Passivation that is successful begins with surgically clean surfaces, is achieved with precise control of process parameters, and concludes with appropriate post-processing and scientific verification.
The seven steps have to be learned and adhered to in a strict manner to ensure stainless steel products attain maximum corrosion-resistance potential and offer long-term value and reliability.
Why Trust This Guide? Practical Experience From LS Experts
For over twenty years, LS Precision has provided precision stainless steel components to over two thousand aerospace, medical, and high-tech manufacturing industry clients. We hold the view that passivation is not just an individual process but a grouped process incorporating material science, chemistry, and surface treatment.
The improved corrosion resistance and reliability of each component we provide depend on tens of thousands of rigorous process verifications and failure lessons learned.
The seven most significant methods outlined here have been refined through experience by LS Precision in real cases. For example, LS Precision accurately forecasted a batch pitting faced by one medical device company as a result of too high a concentration of chloride ions in its wash water. LS Precision also always rectify excessive corrosion as a result of unintended, excessive passivation durations.
These products, removed from the production line and analyzed by science, provide you with the confidence you need to avoid costly, hidden errors and ensure product integrity.
What Is Stainless Steel Passivation? Why Is It A Necessary Corrosion Protection Process?
“Passivated stainless steel, what is it?” — Not a question of rendering it inert, but a necessary chemical follow-treatment that is designed to enhance stainless steel’s natural corrosion resistance.
1. Stainless steel’s ultimate corrosion resistance:
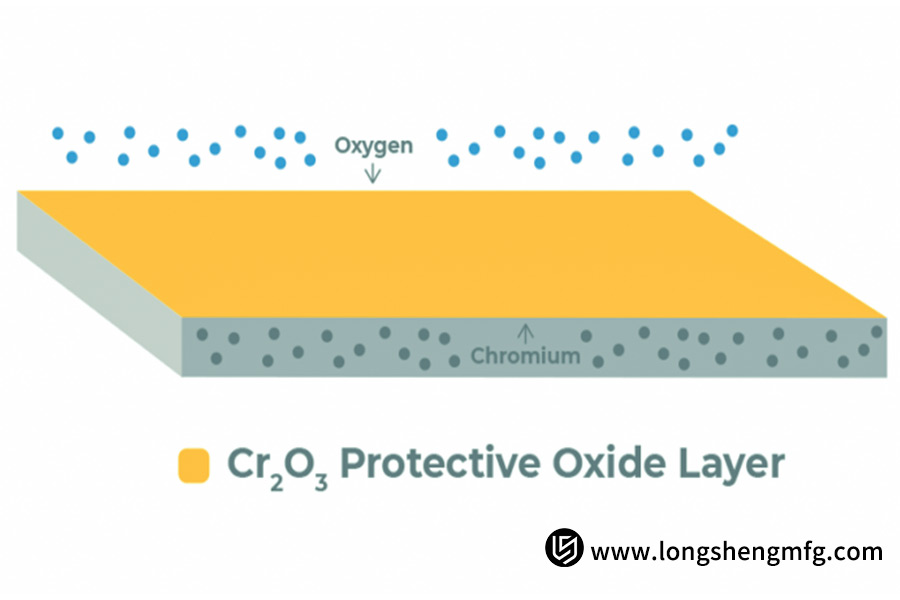
The corrosion resistance of stainless steel is not complete. The key is the chromium (Cr), which oxidizes to form an extremely thin yet dense chromium oxide film (primarily Cr₂O₃) protection layer. The inert “passivation film” insulates the metal from the external environment and thus defends it against corrosion.
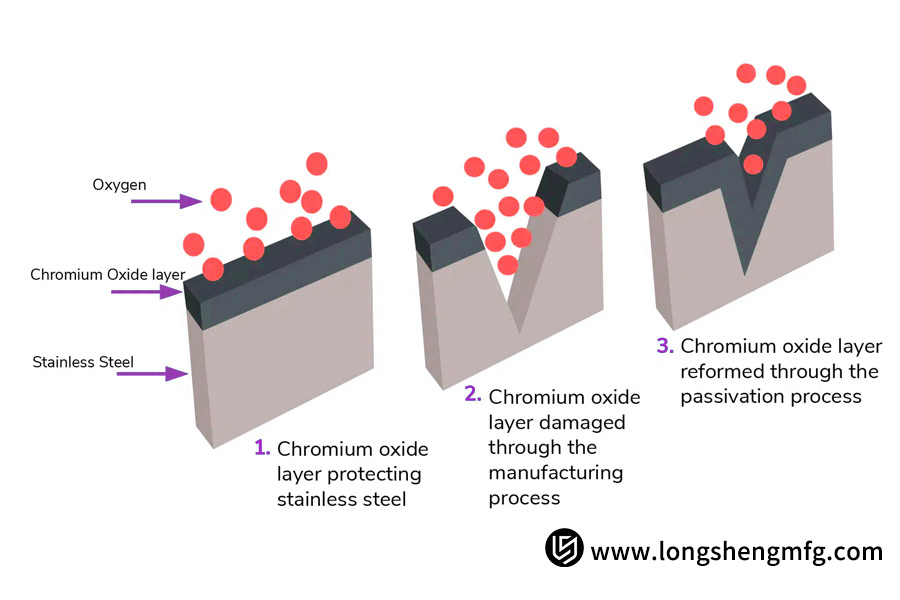
But during welding, machining, or grinding, free iron particles will get lodged in or become stuck in the surface material. These impurities are themselves susceptible to rust and will have a tendency to disrupt the continuity of the chromium oxide film.
Passivation involves chemically dissolving such loose free iron impurities on the surface by immersing the part in a specific acid bath (citric or nitric acid) without compromising the substrate metal underneath.
In so doing, the acid selectively removes the iron impurities and promotes the surface chromium to react with the dissolved oxygen in the atmosphere and re-form a more uniform, stronger, and thicker chromium-rich protective oxide layer.
2. Application of anti-corrosion processes across various industries:
Passivation is this largely imperceptible wall of protection that guarantees the future of stainless steel in corrosive environments. In the medical, food, marine, and chemical industries, a microscopic rust presence can have absolute ruin consequences, ranging from product contamination to equipment or structural collapse.
Passivation is therefore no optional step; it is a critical anti-corrosive treatment to ensure product purity, safety, and longevity, and an absolute pillar of high-stardom excellence in high-quality production.
Passivation is “cleaning” the stainless steel surface chemically and enhancing its natural protective film. It effectively removes potential corrosion risk, thus activating the material’s real durability mechanisms.
Passivation is one of the fundamental technologies that enable critical components to operate at their best state and safely.
How Does Surface Pretreatment Lay The Foundation For Passivation?
Whether passivation is effective or not lies to a large degree at the pretreatment phase. If passivation is comparable to “plating” over an unseen layer of protection, then pretreatment is to have a clean and unblemished substrate on which this layer can be deposited.
1. Pretreatment steps:
System pretreatment is an unforgiving step-by-step process:
- Clean and degrease meticulously first. Oil contaminants must be completely eliminated by a specialist alkaline cleaner or organic solvent.
- This is then followed by critical descaling and damage removal. Heat discoloration (tempering) and oxide scales from welding need to be mechanically removed (e.g., fine sandblasting or wire brushing using stainless steel wire) or chemically pickled (typically with nitric acid-hydrofluoric acid solution) to expose a flat, pure, chromium-rich surface.
Surface roughness must also be closely controlled throughout the entire process. Very rough surfaces are trapbers for impurities and may have corrosion microcells develop, while uniform smooth surfaces encourage a high-density unbroken passive coating.
2. Post-pretreatment cleaning:
Pretreatment cleaning is also necessary. High-purity or deionized water with very low chloride ion concentrations only can be employed in order to prevent secondary contamination. Quality of the pretreatment directly affects the ceiling of surface passive film performance—a nice start and the very basis to achieving good corrosion resistance, process stability, and reliability.
Pretreatment provides ideal conditions for subsequent chemical reactions in a sensitive sequence of operations, a nice start to actually ensure proper passivation and avoid costly corrosion failure.
What Is The Correct Concentration And Temperature Range For Pickling Passivation?
Pickling passivation process parameters by no means remain constant; their very specific configuration directly affects final film quality as well as workpiece safety. Concentration, temperature, and time are chosen primarily based upon the form of passivating chemical available as well as the specific stainless steel material.
1. Nitric acid passivation:
For the most widely used nitric acid passivation, 20%-50% by volume is the suggested concentration for standard austenitic 300 series stainless steels (such as 304 and 316). Working temperature needs to be controlled between 21°C and 60°C, while immersion time should be around 30 minutes.
For martensitic 400 series stainless steels, it is required that a corrosion inhibitor be used and lower concentrations (e.g., 20%-25% nitric acid) and shorter times can be used to prevent excessive corrosion. Nitric acid passivation must not be used on sulfur-free free-cutting stainless steels (e.g., 303).
2. Environmentally friendly citric acid process:
Green process for citric acid has come as a principal substitute for nitric acid. Concentration is typically 4% to 10% by weight, but temperature of operation is higher (typically 60°C to 70°C) and processing time is about 30-40 minutes. It is very safe, easy for waste disposal, and gives very good results on 300 series stainless steel and thus finds widespread use in aerospace, medical, and other industries.
Stringent adherence to the specific pickling and passivation process parameters for specific materials and solutions is the key to success. Operating in ignorance of the correct pickling and passivation process parameters may result in incomplete coating or workpiece damage.
It is essential to adhere to authoritative standards (e.g., ASTM A967) and pre-production process verification.
How To Avoid Common Color Variation During The Passivation Process?
Color variation in the shape of clouding, streaking, or localized darkening after passivation is more than a surface imperfection; it typically shows unstable process control or material defects. Inconsistencies such as these are typically a direct result of microscopic variations in film thickness or composition, which may indicate localized corrosion resistance deficiencies.
The main causes of color variation and their corresponding countermeasures are as follows:
1. Temperature and concentration non-uniformity:
This is the most common cause. Temperature and concentration stratification in a local region can arise from still bath solution. The solution lies in offering the bath a circulating filtration system and heat and cool units so that composition and temperature become extremely uniform throughout the bath, with very strict control of the process parameters within narrow ranges.
2. Unpredictable material condition:
Work pieces from the same lot of different steel mill heats or after different pre-treating (such as welding or heat treating) can have microstructure of surface chemical differences, leading to varying rates of reaction with passivation solution. The best way to prevent this is to batch by material and processing history before passivation, and keep pre-treatment (such as pickling) uniform.
3. Air trapping or shadowing of the workpiece:
When submerged into the bath, denser workpieces or ill-mounted can easily create air bubbles or shadowed areas and induce reaction to some degree. Optimize mounting techniques to ensure solution free flow to all surfaces, and rework workpieces in mid-process where necessary.
Through preserving solution homogeneity, process standardization, and monitoring differences in materials, color differences can be readily eliminated, and a quality passivation film is created with a pleasing appearance and uniform performance.
Why Is Post-Passivation Cleaning So Crucial?
Everyone believes that passivation is complete as soon as the covering paint on the acid container is stripped away. No such thing. Post-washing is an extremely critical component of passivation, and its quality goes a long way toward ensuring the integrity and longevity of the ultimate passivation coating. Insufficient cleaning can make residual passivation solution a new source of corrosion, virtually negating everything accomplished.
1. Rinse water quality:
Rinse water quality is critical, and that of chloride ions is a concern of paramount significance. Chloride ions will easily penetrate the freshly deposited unstable chromium oxide protective layer and cause pitting corrosion.
Deionized or distilled water with extremely low chloride ion concentration (recommended <25 ppm, but a requirement of strict <10 ppm) must be used. In addition, the water pH should be close to being neutral so that it will not end up with extremely acidic or extremely alkaline water that will ruin the film or bring in hydrolytic contamination.
2. Standardized cleaning process:
They would need some rinses for proper cleaning:
- An initial quick rinse from a running water tank where most of the remaining acid is wiped away, then proper cleaning by soaking or spraying.
- In high-grade applications, a further final rinse in boiling deionized water or alkaline neutralizing solution (e.g., sodium carbonate solution) is commonly added to achieve final purity and complete elimination of any residual acid.
Post-rinse is not just a “rinse”; it is an examination required to properly passivate and prevent secondary contamination. Investing in good water and formalized cleaning processes is the cheapest way of protecting a product against hidden corrosion and long-term reliability.

How To Verify Passivation Effectiveness Through Testing?
Passivation is invisible, but its performance has to be assured by visible, scientific means of testing. Passivation quality can only be guaranteed that it is measurable and controllable by providing an effective, standard test system. Nowadays, the industry mostly employs the following standard means of verification:
1. Salt spray test:
A rapid corrosion test that puts the complete protective performance of the passivation film under severe testing. The test material is placed in a salt spray chamber with controlled temperature and constantly sprayed by 5% sodium chloride solution for more extreme simulation.
Acceptance standards typically have their basis in specifications (e.g., ASTM B117). For example, 304/316 stainless steel would normally be specified as having a minimum of 96 hours (4 days) of zero red rust. It is the most severe and effective test method.
2. Copper ion test (Copper Sulfate Spot Test):
Rapid, qualitative field test procedure. Place a drop of solution of copper sulfate on the surface of the washed specimen. If free iron or in the absence of passive film is present, the iron displaces the copper ions in the solution to produce a red copper-colored deposit evident. Acceptance criteria are no indication of any red copper-coloured deposits within the specified observation time (typically 5-6 minutes).
3. Potassium ferricyanide test:
This is used for specifically determining free iron contamination on a surface. Potassium ferricyanide and nitric acid solution is dripped on the surface. On the occurrence of free iron particles on the surface, a characteristic “Teng’s Blue” precipitate will be produced immediately. Acceptance requirements are all no blue spots.
The test frequency must adhere to the standards of “mandatory first-article inspection, batch sampling, and periodic audits” for process stability.
The above process of verification and testing can convert the intangible quality of passivation into tangible terms, effectively monitoring process performance, preventing release of faulty products, and finally determining the long-term corrosion resistance and reliability of the product.
LS Case Study: How To Solve The Passivation Failure Problem For A Medical Device Company?
1. Client Challenge:
A well-known medical device manufacturer wrestled with a critical stainless steel passivation problem: tiny rust spots continued to mysteriously appear on the company’s surgical instrument components soon after they rolled off the production line, causing repeated customer complaints and the threat of a batch recall.
Initial in-house quality testing could not find the cause, and although the passivation process appeared to conform to standard operating procedures, the quality problem continued, shutting down production.
2. LS Precision’s innovative solution:
At the customer’s request, LS Precision‘s technical staff went beyond the passivation process itself and performed a root-to-end review of the entire process train from raw material through final cleaning.
With the assistance of modern surface analysis methods (e.g., SEM-EDS), we were able to find the underlying cause in a jiffy: the level of chloride ions in the last rinse water was much too high above the recommended specification (up to 150 ppm). Small amounts of chloride ions were retained on the workpiece surface during drying following passivation, slowly deteriorating the fresh passivation layer and resulting in hidden pitting corrosion.
In line with the above, we never recommended substituting the passivation solution. Instead, LS Precision pushed for a complete new approach:
- LS Precision designed and purchased a special two-stage reverse osmosis pure water system to keep the composition of chloride ion in cleaning water to a very high level of <5ppm at all times.
- LS Precision also employed a complete quality control system, e.g., daily water quality check, passivation solution titration, and regular salt spray test, to ensure traceability and controllability of all parameters.
3. Final outcome:
When the client applied this solution, their medical device parts all passed the 96-hour neutral salt spray test at first try, eliminating the rust problem completely. Not only did this save them from massive recall costs, but it also fundamentally enhanced their products’ reliability and business reputation.
Most significantly, through the implementation of LS’s lean quality management model, the company established a preventative, long-term guarantee mechanism, ultimately increasing the passivation pass rate over 99.5% from below 80%, allowing sustainable high-quality output.

Seven Essential Passivation Best Practices
To achieve steady and uniform stainless steel passivation outcomes, strict control over the entire process should be implemented. The following seven indispensable best practices provide direct actionable guidelines:
1. Stringent solution management:
Conduct automatic testing and solution refreshing on a regular basis, concentration established through chemical titration to avoid a loss in batch quality through ageing or contamination of solutions.
2. Standardize process parameters:
Set such key parameters as temperature, time, and concentration as unbeatable process disciplines, and use automatic equipment to eliminate operating fluctuations.
3. Water quality is a critical control point:
Daily monitoring measurements should be the concentration of chloride ion of cleaning water (<25ppm recommended). Provision of a pure water system binds the root cause of the problem.
4. Extensive pretreatment:
“Oil-free, dust-free, and scale-free” passivation pretreatment requirements as a basis for success with a clean and stable metal substrate.
5. Avoid cross-contamination:
Set aside a dedicated area and equipment for stainless steel processing without mixing with carbon steel equipment to prevent cross-contamination of iron particles.
6. Develop a system of verification:
Implement processes for quality control such as salt spray test or copper ion testing as standard quality control procedures, using statistics for traceability of passivation outcomes.
7. Staff training:
Provide operators with frequent theoretical and practical training so that each step and its meaning is well comprehended, avoiding empirical mistakes.
To master these seven practices will consistently improve passivation quality consistency and significantly reduce the risk of corrosion.
FAQs
1. Do all stainless steels need to be passivated?
Not all stainless steels do. If the material is in its unbroken, passive state and unprocessed, nothing needs to be done. Mechanical processing like cutting, welding, and grinding can strip the surface passivation layer, however, and in doing so potentially bury free iron particles. Passivation is thus warranted to eliminate impurities and to restore the protective film of chromium oxide, recovering the desired corrosion resistance.
2. Are further protective measures needed after passivation?
Passivation is a significant treatment that enhances the inherent corrosion resistance of stainless steel and is typically sufficient. In highly corrosive environments (e.g., high chloride ion content) or where cleanliness will need to be very high (e.g., biopharmaceutical), such treatment may be done as a base coat with supplements such as electropolishing to further reduce surface roughness and maximize the efficiency of the passivation film to create a more comprehensive protective system.
3. How do I regulate whether replacement of the passivation solution is necessary?
It relies primarily on periodic chemical examination. When the metal ion concentration of the solution (especially iron ion concentration) is excessively high or the pH level is quite unequal, it indicates that too much active substances have been consumed and the passivation effect has been destroyed. Even when passed, in order to have the same quality, a compulsive replacement cycle dependent on the process volume is proposed to ensure batch-associated quality risks are avoided.
4. How can small businesses achieve an economical passivation system?
LS Precision can provide low-cost passivation modules to meet your product volume and price. These encompass recommendations for appropriate tank sizes, testing equipment needed, and user-friendly process procedures so that firms can establish a compliant passivation manufacturing facility at economic cost. Master operation training is also provided to install the system instantly and efficiently.

Conclusion
Choosing professional passivation services assures unparalleled quality consistency. By utilizing a tested process system along with stringent quality control, it practically eliminates batch corrosion, rework, and even recall risks caused by improper in-house processing.
Professional passivation services also cost-effectively reduce overall costs, allowing you to avoid costly investments in equipment, chemicals, testing, and wastewater treatment, so you can keep internal resources free for core infrastructure manufacturing.
For maximum product reliability and best value, contact LS Precision the surface treatment experts. LS Precision Manufacturing experts will provide a complimentary passivation process analysis and upgrade recommendation so you can tailor the most cost-effective solution.




Disclaimer
The content appearing on this webpage is for informational purposes only. LS makes no representation or warranty of any kind, be it expressed or implied, as to the accuracy, completeness, or validity of the information. Any performance parameters, geometric tolerances, specific design features, quality and types of materials, or processes should not be inferred to represent what will be delivered by third-party suppliers or manufacturers through LS’s network. Buyers seeking quotes for parts are responsible for defining the specific requirements for those parts. Please contact to our for more information.
Team LS
This article was written by various LS contributors. LS is a leading resource on manufacturing with CNC machining, sheet metal fabrication, 3D printing, injection molding,metal stamping and more.





